Regenerative Medicine and Surgery with Placental/Amniotic Allograft
Placental/Amniotic products are regulated by the FDA under 21 CFR Part 1271 Part 361 Human Cells, Tissues. and Cellular and Tissue-Based Products (HCT/Ps).
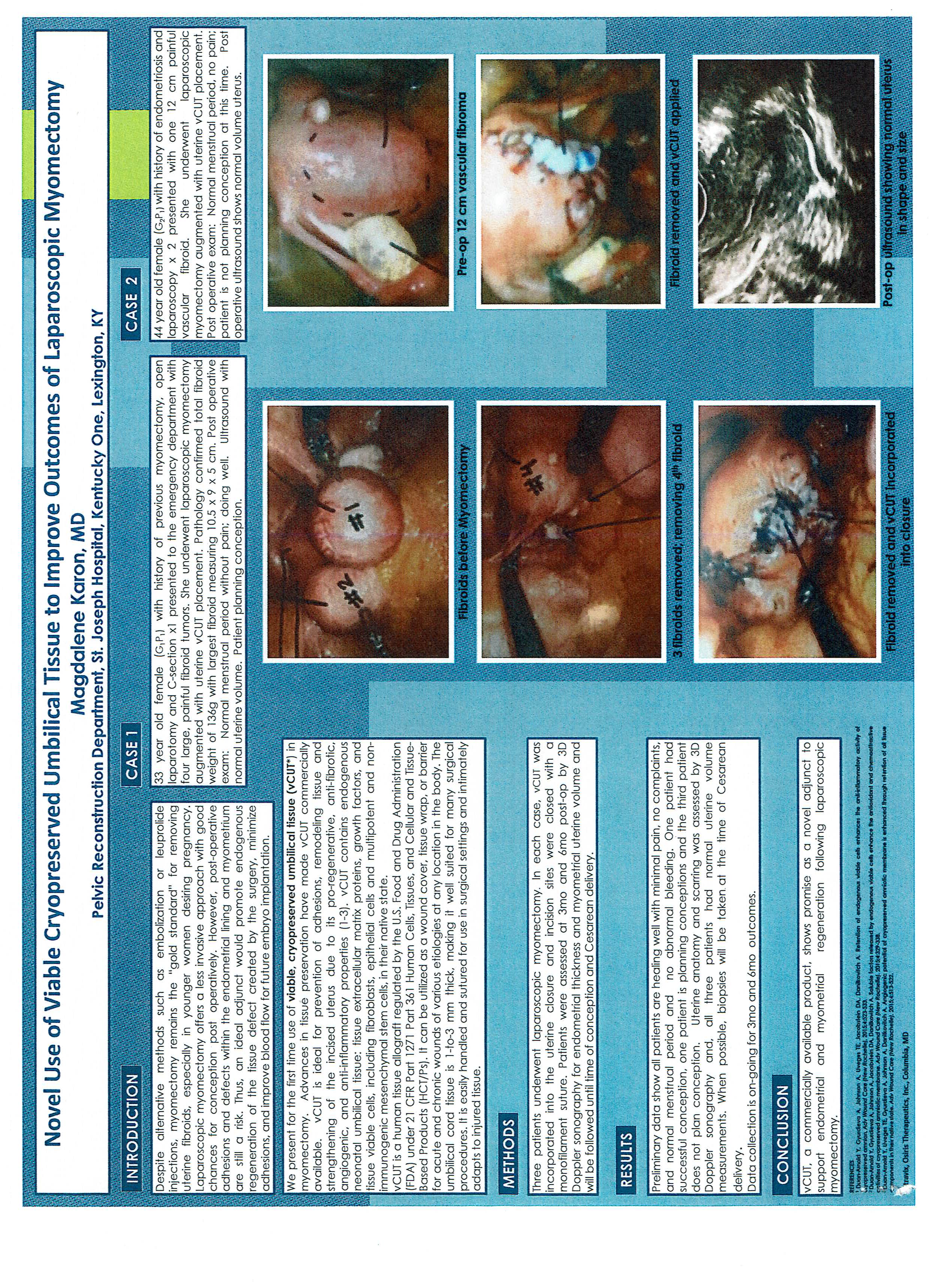
Dr. Karon is the first one in her specialty to use donor amniotic fluid, umbilical cord and placental membranes from tissue banks. As a urogynecologist and one of the busiest robotic surgeons in Kentucky, she uses it to complement her pelvic prolapse surgery without mesh. One of the other applications is as an adhesion barrier for adhesion prevention during surgery especially with endometriosis patients. Another application is myomectomy. Anti Mullerian hormone has been shown to rise after the use of amniotic fluid during laparoscopic surgery as a measure in improving ovarian reserve. This was discovered and then published by Dr. Karon in the ACOG official journal in April 2018. Other applications are being developed as she works in concert with scientists and PHDs.
To date the FDA has had no reported complications from the use of native “birth tissue” as long as the guidelines issued by the FDA for the homologous use are followed. The use of placental tissue dates back to Egyptians days.
https://journals.lww.com/greenjournal/Abstract/2018/05001/Restoring_Function_with_Neonatal_Fluid_Allograft.665.aspx